Purposeful Agility: Mastering the Art of Doing What Matters in Pharma
“There is nothing so useless as doing efficiently that which should not be done at all.” – Peter Drucker
This quote from Peter Drucker cuts to the heart of a persistent challenge in the pharmaceutical industry: the temptation to optimise processes, streamline operations, and chase efficiency metrics - all while pursuing the wrong goals. In an era of rapid scientific advancement, regulatory shifts, and market volatility, pharma companies are on a track to, but can't afford to, excel at irrelevance.
Instead, we need a framework that balances execution with forward ideation, introspection, and adaptation. Enter Purposeful Agility, a concept we've been refining at IDEA Pharma since 2020.
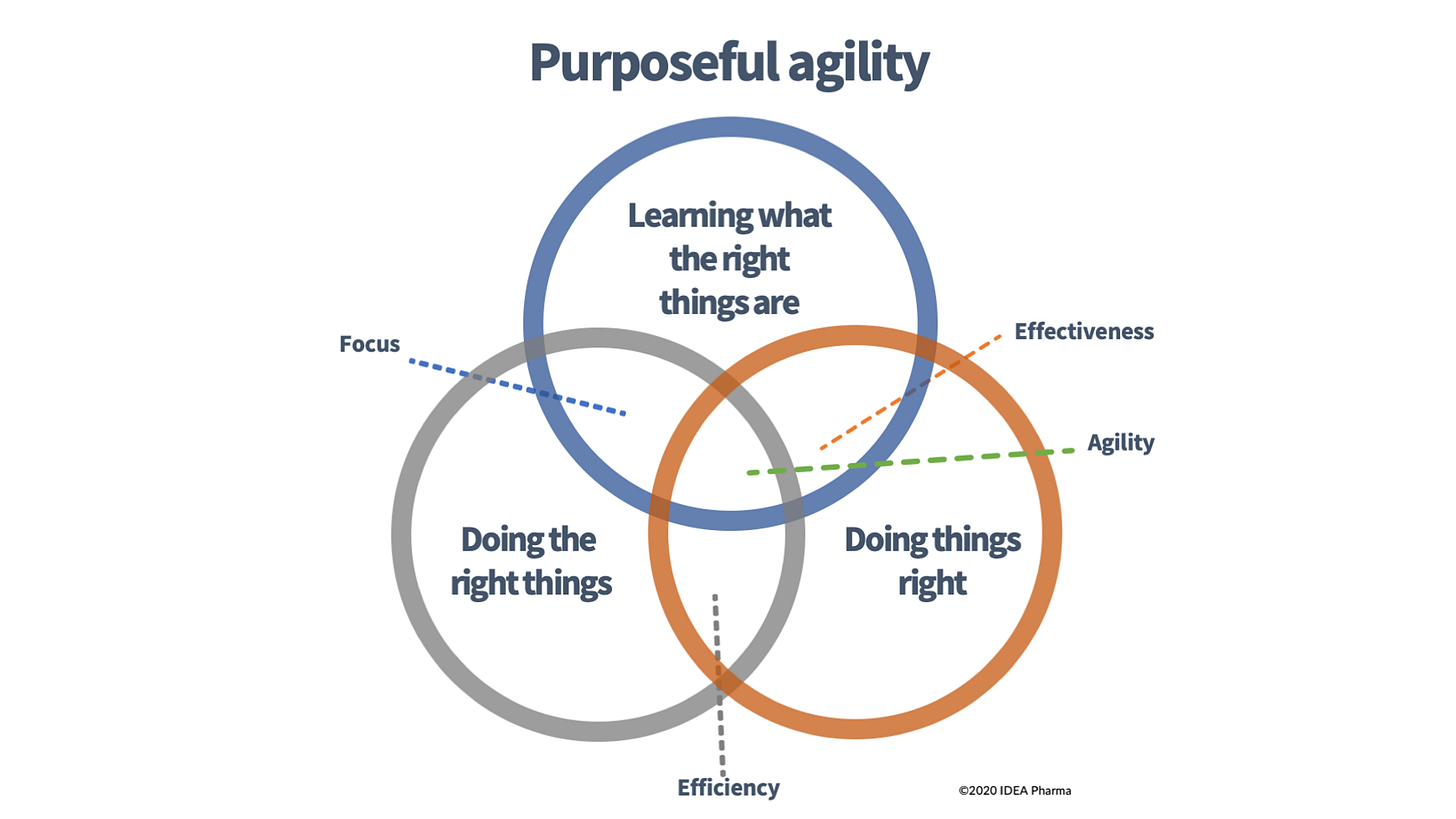
At its core, Purposeful Agility is visualized in this diagram:
The Three Pillars of Purposeful Agility
Doing the Right Things (Efficiency)
This pillar represents the foundation of focus: identifying and prioritising activities that truly drive value. In pharma, efficiency often gets misconstrued as cost-cutting or speeding up timelines (typically, time to phase transition) without questioning the underlying strategy. Think of it as the grey circle in the diagram - solid but static if isolated. Companies that excel here might avoid wasting resources on dead-end projects, like pouring millions into a drug candidate with flawed market fit. But without the other elements, you risk becoming a well-oiled machine churning out mediocrity.Doing Things Right (Effectiveness)
The orange circle embodies execution excellence: optimising how we deliver on our chosen paths. This is where R&D teams shine in clinical trial design, manufacturing scalability, or go-to-market strategies. Effectiveness ensures that once we've picked a target, we hit it with precision. In oncology, this means refining biomarker-driven trials to boost success rates. Yet, as Drucker warns, perfecting the wrong approach is futile - like accelerating a car headed off a cliff.Learning What the Right Things Are (Agility)
The blue circle at the top is the dynamic force that we’ve added to the classic two-circle diagram: continuous learning and adaptation - asymmetric learning at its core. Agility isn't about pivoting wildly; it's purposeful iteration based on data, feedback, and emerging insights. In the diagram, it overlaps with the others to create balance. Pharma examples abound: AstraZeneca's pivot in oncology toward precision medicine, or Lilly's agile structure that combines big-pharma resources with startup-like focus. This pillar demands skepticism, experimentation, and a culture that embraces uncertainty - core values we've discussed in previous posts like "Core Values: Skepticism and Unbiased Experimentation."
The magic happens in the overlaps. The intersection of all three? Purposeful Agility - a state where efficiency, effectiveness, and learning reinforce each other.
Focus emerges from aligning what we do with why we do it; agility ensures we adapt as the landscape evolves.
Why Pharma Needs Purposeful Agility Now More Than Ever
In 2025, the industry faces unprecedented pressures: AI-driven disruptions, supply chain vulnerabilities, and the quest for breakthroughs in areas like oncology. Large companies often struggle with inertia, as highlighted in "The Large and the Agile," where we explored how major players like Lilly maintain nimbleness through hybrid models. Meanwhile, smaller players risk inefficiency without the scale to learn quickly.
Consider a real-world trap: A company efficiently develops a "me-too" drug, hitting all milestones on time, only to launch into a saturated market. That's efficiency without agility - useless perfection. Contrast this with asymmetric learning: Using AI to analyze trial data in real-time, questioning assumptions, and quitting non-viable paths early (as Annie Duke advises in her book Quit, which we unpacked in a recent episode). To implement Purposeful Agility:
Foster a Learning Culture: Encourage true cross-functional teams to challenge the status quo, as in "The Path to 100%" where feedback loops turn good into great.
Measure What Matters: Track not just speed, but impact - pipeline value, patient outcomes, and adaptability metrics from our Pharmaceutical Innovation Index.
Experiment Boldly: Start small, like piloting agile manufacturing to respond to global disruptions.
Final Thoughts
Purposeful Agility isn't a buzzword/ phrase; it's a deliberate choice to avoid Drucker's pitfall. By centering our efforts on learning, execution, and focus - as illustrated in the diagram - we can transform pharma from a risk-averse machine into an innovative force.